Ensuring CAR T Cell Quality: Validating a Potency Assay to Current Regulatory Standards
CAR T cell therapies are living drugs that use genetically modified T cells to recognize and attack cancer cells. A critical component for ensuring the efficacy of these therapies are potency assays that measure CAR T cells’ efficacy in killing their target cells such as transformed B-cells in the case of Yescarta and Kymriah. The paper “In vitro CAR-T cell killing: validation of the potency assay” focuses on validating such an assay to meet current FDA regulatory guidelines. Validation is crucial for quality control because inaccurate potency assessments could lead to ineffective and/or unsafe treatments. This study serves as a good demonstration of the experiments needed for potency assay validation and highlights the importance of robust and reliable methods for CAR T cell quality control.
CAR T Cell Therapy for Cancer Treatment and Beyond.
Chimeric antigen receptor (CAR) T cell therapy represents a groundbreaking approach to personalized medicine such as cancer treatment by harnessing the power of the adaptive immune system to restore the function of endogenous immunity or to kickstart an immune response through synthetic immunity. CAR T cells are made with isolated patient T cells, where the CAR gene that enables T cells to recognize specific epitopes and attack target cancer is introduced. The CAR T cells are expanded to quantities needed for dosing a patient (Figure 1). Each step is carefully documented and tested for quality control.
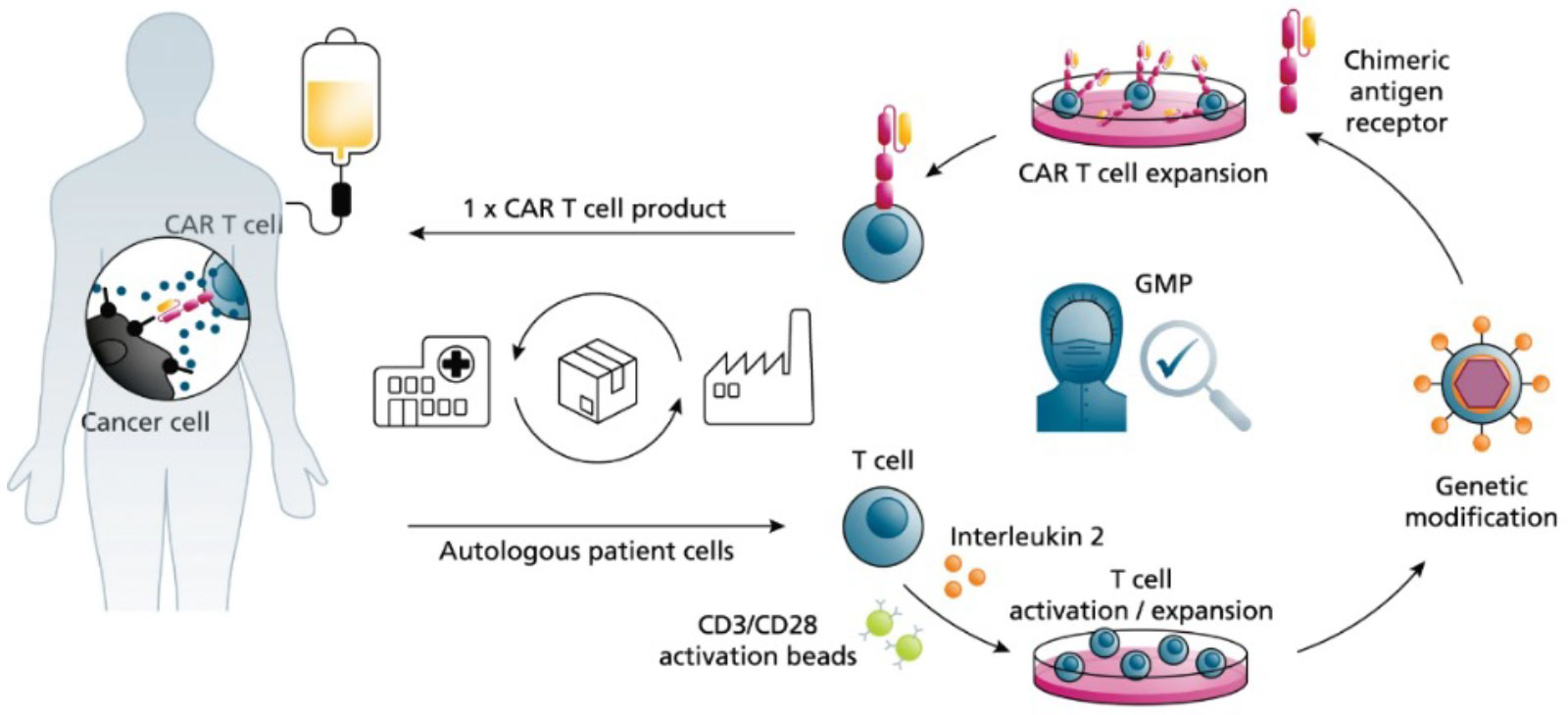
Figure 1: Generic CAR T Cell Production Process. Adapted from Blache et al. 2022.
CAR T cell therapy has been remarkably successful in treating certain hematological cancers, eliciting long-term remissions in patients with previously refractory disease. Building on its clinical success, the field has rapidly grown to six FDA approved and commercially available CAR T therapies treating blood cancers (Table 1). Many additional CAR strategies are in clinical testing for a range of indications including autoimmune diseases like lupus.
Table 1: FDA-approved CAR T cell therapies.
| Therapy Name | Target Antigen | Approved Indications | Manufacturer | Approval Date |
|---|---|---|---|---|
| Kymriah (tisagenlecleucel) | CD19 | B-cell acute lymphoblastic leukemia (ALL) and B-cell non-Hodgkin lymphoma | Novartis | August 2017 |
| Yescarta (axicabtagene ciloleucel) | CD19 | B-cell non-Hodgkin lymphoma, follicular lymphoma | Kite Pharma Inc | October 2017 |
| Tecartus (brexucabtagene autoleucel) | CD19 | Mantle cell lymphoma, B-cell acute lymphoblastic leukemia | Kite Pharma Inc | October 2021 |
| Abecma (idecabtagene vicleucel) | BCMA | Multiple myeloma | Bristol Myers Squibb | March 2021 |
| Carvykti (ciltacabtagene autoleucel) | BCMA | Multiple myeloma | Janssen Biotech, Inc. | February 2022 |
| Breyanzi (lisocabtagene maraleucel) | CD19 | B-cell non-Hodgkin lymphoma | Bristol Myers Squibb | March 2024 |
Robust Potency Testing is Critical for Product Safety and Efficacy
Since approval in 2017, regulatory agencies including the FDA in the US and EMEA in Europe have published industry guidelines for the quality control of CAR T cell products. Central to this framework is the assessment of potency, which measures a product’s ability to achieve its intended biological effect. In the context of CAR T cells, this can include target cell killing, cytokine production, and other mechanisms of action. Ultimately, CAR T cell potency testing should include five critical assessments (1) cell viability, (2) vector transduction efficiency and (3) CAR expression, (4) cytotoxicity and (5) persistence. This is complimentary to identity testing, which confirms the product’s characteristics and composition. Both the FDA and EMA recommend various bioassays to assess each of the attributes listed above. Lacking a fully developed potency assay assurance strategy could lead regulatory agencies to place an IND application and/or commencement of a phase 2 clinical trial on hold. To ensure proper assay performance and avoid commercial delays, potency assays must be validated according to regulatory standards. Potency validation experiments include testing for specificity, linearity, accuracy, and precision. Ultimately, validated potency assays are critical for understanding and evaluating the therapeutic potential of CAR T cell products.
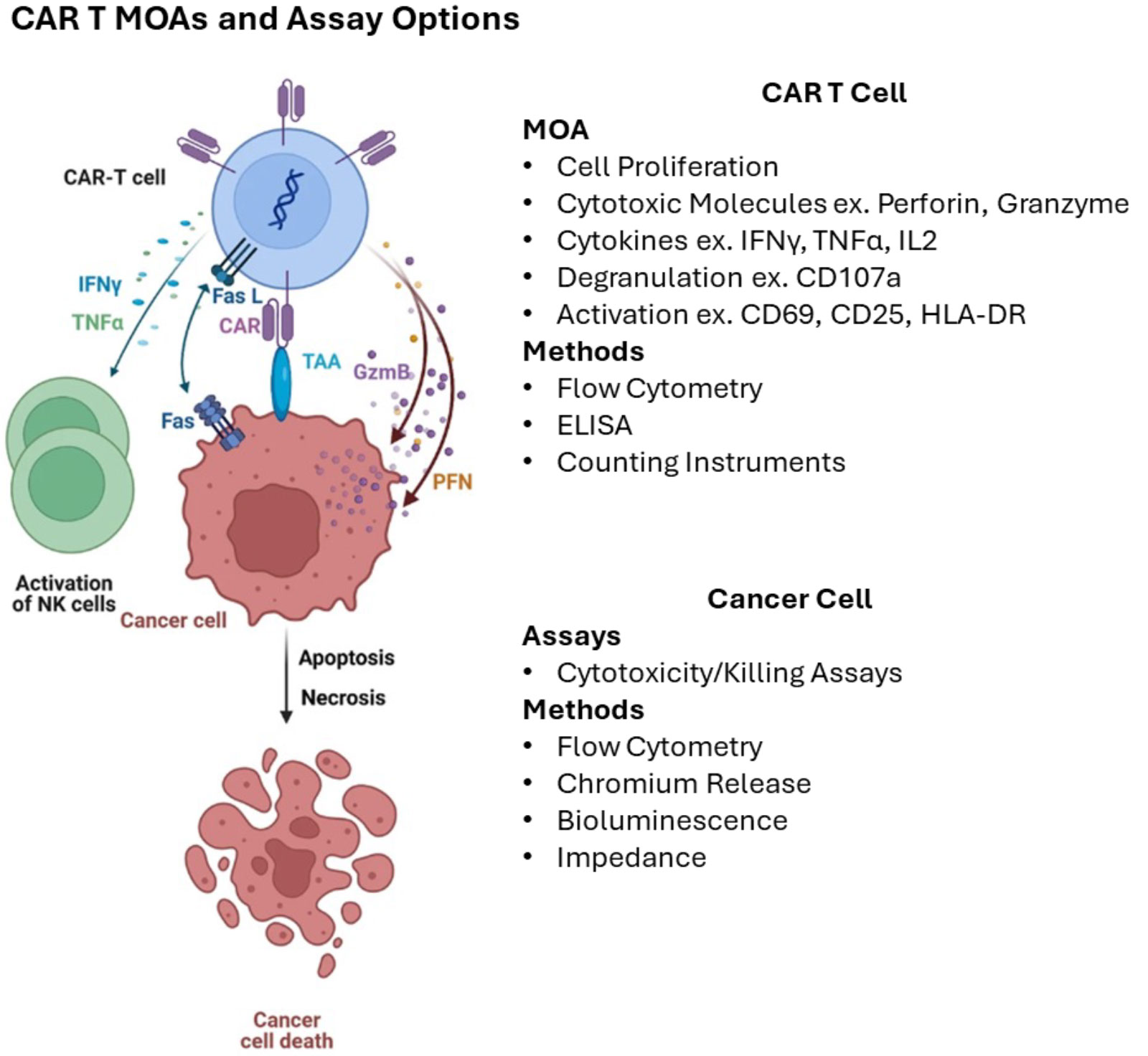
Figure 2: General mechanisms of CAR T cell killing and assay methods for measurement. Adapted from Maalej et al. 2023.
Killing Assays for Measuring Potency
The biological effect of CAR T cells involves complex interactions between the CAR T cell, the target (ex. cancer cell), and the patient’s immune system. From the FDA’s Guidance on Potency, potency assays “… should be developed based on your understanding of the product’s mechanism of action (MOA), the intended clinical indication, and the route of administration”. Prior knowledge and product characterization, preclinical and clinical studies may be used to establish a clear relationship between the potency assay and the product’s mechanism of action.
Co-culture cytotoxicity or killing assays are common potency assays in the CAR T cell space. This format involves incubating live CAR T cells with live tumor cells that express the CAR’s target epitope and measuring target cell death by direct or indirect methods after a specified duration. Common methods for measuring killing include chromium release (currently the gold standard), bioluminescence, impedance, and flow cytometry (Table 2). Assays vary in the requirement for materials, equipment, control or reference material, and time (Table 5). Assays also offer different benefits like the ability to capture data over time or scale-up for large numbers of samples. The choice of assay(s) depends on the specific product and its intended therapeutic effect. There are currently few commercial standards or control material to support CAR T cell killing assays, and companies often develop these materials in-house.
Table 2: Attributes of different CAR T cell killing assay formats.
| Assay format | Chromium release | Bioluminescence | Impedance | Flow cytometry |
|---|---|---|---|---|
| Principal measure of cytotoxicity | 51Cr release | Luciferase activity | Cell detachment | Live/dead staining, phenotype |
| Radioactive materials needed | Yes | No | No | No |
| Target cell labeling required | Yes | No | No | Yes |
| Genetic modification of target cells required | No | Yes (reporter gene) | No | No |
| Endpoint/kinetic | Endpoint | Endpoint | Temporal | Endpoint |
| Real-time measurement | No | No | Yes | No |
| Maximum time point measured | 18–24 hours | Days | Days | Days |
| Ability to measure differential cytotoxicity on heterogenous targets | No | No | No | Yes |
| Throughput and automatability | Low | High | High | High |
Validating a CAR T Potency Assay
A recent study by Piccinini et al. published in Cancer Immunology, Immunotherapy in June 2024 provides a nice example of CAR T cell potency assay validation. The authors developed a co-culture cytotoxicity assay using flow cytometry to measure killing of CD19+ tumor cells by CD19 CAR T cells. In brief, the CD19 CAR gene was introduced to isolated T cells using a lentivirus vector. CD19+ CD3+ CAR T cells were confirmed by identity testing using flow cytometry. Co-culture experiments with engineered CD19+ target tumor cells were analyzed at 6-, 24-, and 68-hour time points and a series of effector:target ratios from 1:1 to 5:1. At the end of each time point the amount of dead 7-AAD+ CD19+ target tumor cells was measured by flow cytometry.
An initial step in assay validation is choosing appropriate acceptance criteria used to determine conformance to specification. Acceptance criteria are numerical limits or ranges and should be chosen based on data from the development process including preclinical and clinical testing, manufacturing lots and stability studies where no prior guidance from scientific literature is available. For example, in Piccinini et al., the authors used the acceptance criteria listed in Table 3 Implementing reference material and including the proper controls is critical to support quality and guide data interpretation.
Table 3: Acceptance criteria for CAR T validation assays. Adapted from Piccinini et al.
| Assay | Acceptance Criteria |
|---|---|
| Intra-assay | CV = 10% |
| Inter-assay | CV = 20% |
| Inter-day | CV = 20% |
| Inter-analyst | ICC > 0.4 |
| *CV – coefficient of variation, *ICC – intra-class correlation coefficient | |
The authors included two main controls: an off-target CD19- tumor cell line to assess specific killing of target CD19+ cells, and isolated T cells that did not express the CD19 CAR to assess killing meditated specifically by the CAR.
Once the killing assay was optimized, properly controlled, and the acceptance criteria determined, the authors evaluated specificity, linearity, accuracy, and intra- and inter-assay precision to validate the killing assay (Table 4).
Table 4: Experiments for assay validation and their definition (See ICH Scientific Guideline).
| Validation Experiment | Definition |
|---|---|
| Linearity | Measurement of diluted analyte over a certain operational range. |
| Accuracy | Closeness of agreement between expected value and the measured value. |
| Precision: Repeatability/Intra-assay | Closeness of agreement between series of measurements from multiple tests of same sample. |
| Precision: Intermediate/Inter-assay | Closeness of agreement between series of measurements from multiple tests of same sample made over multiple days or by different operators. |
| Range | Lowest and highest concentrations that produce acceptable accuracy, intermediate precision and linearity. |
| Specificity | Detection of analyte in the presence of matrix, impurities etc. |
| Robustness | Systematically testing experimental variables and confirming assay performance. |
Specificity is the ability for the assay to accurately detect what it was intended to, without interference from other substances or factors in the experiment. Here, the authors co-cultured CD19 CAR T effector cells with CD19+ target or CD19– off-target cells and observed significant killing of only CD19+ target cells.
The goal of linearity experiments is to demonstrate that the assay measurements are directly proportional to the amount of analyte in the measured sample. Linearity experiments involve diluting the measured analyte over a certain operational range and correlating the measurement with the expected value. Here, the authors ran the CD19 effector:target co-culture at a 1:1 ratio. After 24 hours in culture, fresh live CD19+ target cells were added at different amounts and measured immediately by flow cytometry, effectively diluting the 7-AAD+ dead target cells for analysis. The correlation coefficient was calculated and met the predetermined acceptance criteria indicating good agreement between measured and expected amounts of killing.
Accuracy experiments confirm that the assay measures the true or known value of an analyte in any given sample and are required by the FDA for assay validation. Scientists will often look to vendor supplied reference material with characterized or known quantity of target analyte. The measured value from the assay being validated can then be compared to the manufacturer’s specifications. Accuracy is challenging for killing assay validation, because the complexity and heterogeneity of CAR T cell products makes development of standard or control material difficult. There are currently no widely used control materials for CAR T cell killing assays and companies often create their own in-house.
To deal with a lack of “true” value for accuracy, the authors performed killing assays with three independent batches of CAR T cells, where CD19+ target cells were added at different dilutions. The expected killing was calculated as the mean killing in the undiluted sample multiplied by the proportion of target cells to effector. The measurement of dead cells by flow cytometry was then compared to the expected values and to a trypan blue dead cell counting method. The acceptance criteria was met with ≥90% accuracy.
Though not explicitly required by regulatory agencies, assay robustness assesses assay performance given variations in experimental conditions like time, temperature, and reagent concentration. Here the authors showed that the assay was resistant to 1-hour variations in co-culture incubations time, as the %CV was less than 10% whether the incubation time was 23-, 24-, or 25-hours.
Precision for validation is the measure of agreement among a series of measurements of the same sample under different conditions. Precision assays can be categorized depending on the variable that is being tested. Intra-assay precision is also referred to as repeatability and measures the agreement between runs using the same sample, on the same day, by the same operator. In their study, the authors measured the %CV for intra-assay precision as less than 3.5%. Inter-assay precision, or intermediate precision, measures the agreement between runs of the same samples but considering measurements on different days, performed by different operators, and even on different instruments. In this study, the authors considered three runs on the same day by the same operator, three runs on different days by the same operator and three runs by different operators for the inter-assay precision evaluation. Their results were within the predetermined %CV threshold, indicating that the assay is precise.
By following these validation steps, the authors established a reliable potency assay for CD19-targeted CAR T cells. This case study highlights the importance of rigorous experimental design and data analysis in ensuring the quality and consistency of CAR T cell potency assessments.
Challenges and the Road Ahead:
Developing standardized potency assays for CAR T cell therapies presents significant hurdles. The complex and heterogeneous biological activity of these products can be mediated by multiple factors and may be difficult to capture using a single potency assay. This also renders establishing reference standards and control materials challenging. Reference standards themselves pose challenges like re-qualification and comparability of new lots and even revalidation of the assay if it is not possible to generate comparable reference material. All current FDA-approved CAR T cell products are autologous, although there are several therapies in clinical trials that are allogeneic. Sample availability for conducting a wide range of tests might be limited from some patients and some products are administered fresh, limiting the time available for potency testing. Factors such as tumor cell line variability, assay complexity, and associated costs further complicate the process. Despite these challenges, the field is making progress.
Regulatory agencies like the FDA and EMA have recognized the need for clear guidance and have published documents outlining the requirements for potency assay validation:
- FDA Potency Assurance for Cellular and Gene Therapy Products
- EMA Potency Testing Scientific Guideline
- FDA Test Procedures and Acceptance Criteria for Biological Products
- FDA Validation of Analytical Procedures
- ICH Validation of Analytical Procedures Scientific Guideline
These guidelines provide a framework for developers to follow when designing and implementing their assays. The research by Piccinini et al. offers a valuable example of a well-conducted potency assay validation study, demonstrating best practices for assay development and execution.
As the field of CAR T cell therapy continues to advance, there is a growing need for novel methodologies and standardized protocols to improve potency assay reliability and comparability. By addressing these challenges and leveraging emerging technologies, the industry can develop more robust and predictive potency assays to support the development of safe and effective CAR T cell therapies.
Accellix For Flow Cytometry-Based Potency Assays
Accellix’s automated flow cytometry platform provides a comprehensive suite of potency assay solutions for CAR T cell quality. These include customizable assays to measure transduction efficiency, viability, immunophenotype, and frequency of T cells expressing the CAR gene. In communication with the FDA Center for Biologics Evaluation and Research, it was confirmed that extracellular markers associated with T-cell and CAR-T activation can be used “… as a measure of potency, if shown to correlate with biological activity during drug development*”. Accellix can also inform on persistence through the measurement of memory CAR genes. While we currently do not measure intracellular cytokine production, this capability is under active development by our R&D team.
Conclusions
Potency assessment is a critical component of CAR T cell product development and manufacturing. While challenges persist in establishing standardized potency assays, significant progress has been made in defining the regulatory landscape and best practices for assay development and validation. The availability of guidance documents from regulatory agencies, coupled with examples of successful assay implementation, provides a strong foundation for the industry.
By adhering to rigorous validation principles, including specificity, linearity, accuracy, and precision, developers can increase confidence in the potency of their CAR T cell products. As the field continues to evolve, the development of novel methodologies and standardized approaches will be essential for advancing CAR T cell therapy and improving patient outcomes.
Validating a potency assay to regulatory standards is a key pillar in the journey to commercialization. To learn more about Accellix’s validation services, reach out to info@accellix.com.
Table 5: Different types of potency assays and their descriptions.
| Assay | Investigation of focus | Description | Recommended assay(s) |
|---|---|---|---|
| Antigen stress test | Adaptive resistance, cytotoxicity following functional exhaustion | Repeated antigen stimulation by multiple rounds of target cell addition to investigate effector cell cytotoxicity under high antigen stress | 51Cr, BLI, impedance, or flow cytometry assay* |
| Differential cytotoxicity on target cells with heterogeneous antigen expression | Cytotoxicity on target cells with antigen heterogeneity (low and high), on-target/off-tumor toxicity on normal cells with very low antigen expression | Cytotoxicity on target cells with variable antigen expression to investigate the correlation between antigen density and cytotoxicity and any unanticipated cytotoxicity of activated effector cells on normal cells with very low antigen expression | If target cells with different antigen expression levels are assessed individually (one cell type per well): 51Cr, BLI, impedance, or flow cytometry assay. If target cells are plated as mixture: flow cytometry assay. |
| Multi-CAR cytotoxicity | Antigen heterogeneity, antigen escape | Cytotoxicity assessment of effector cells with multiple CAR constructs targeting different antigens simultaneously | 51Cr, BLI, impedance, or flow cytometry assay |
| Cytotoxicity in the presence of soluble factors | Immunosuppression by soluble factors known to be present in the tumor environment | Cytotoxicity in the presence of various doses of single or multiple inhibitory or excitatory cytokines and/or soluble factors | 51Cr, BLI, impedance, or flow cytometry assay |
| Cytotoxicity in the presence of immune cells | Immunosuppression by immune cells known to be present in the tumor immune environment | Cytotoxicity in the presence of various ratios of single or multiple immunomodulatory immune cells | Flow cytometry assay to investigate cytotoxicity against heterogenous (target) cell populations |
*This communication is consistent with 21 CFR 10.85 (k) and constitutes an informal communication that represents my best judgment at this time but does not constitute an advisory opinion, does not necessarily represent the formal position of FDA, and does not bind or otherwise obligate or commit the agency to the views expressed.
